Biography
Biography: Rana A Bilbeisi
Abstract
Knots and links are topologically fascinating entities that have been extensively explored in various scientific fields. On a molecular level, chemists developed various synthetic approaches for the preparation and characterization of knots and links. Beyond their aesthetically appealing structures, chemists explored the application of molecular knots and links as molecular machines and switches. The application of exploiting molecular recognition properties of knots and embedding them into polymeric material are areas that are still not well investigated. Topologically unique cavities of knots and links are expected to render their molecular hosts with interesting binding capabilities. Furthermore, based on the properties of naturally occurring and synthetic knotted fibers, knot-based extended networks are expected to be robust yet elastic, and are also expected to serve the material science, biomedical and chemo-sensing fields. We reported a study where anion-binding properties of previously prepared cationic Zn(II)-based trefoil knot (TK6+) and Solomon link (SL8+) were investigated in aqueous solutions. In the solid state, the central cavity of TK6+ hosts two bromide ions through the formation of six aromatic CH anion non-classical hydrogen bonds. In solution, TK6+ retains the property of binding monovalent anions, as evidenced though NMR titrations. Despite carrying out the study in competitive solvent, water, monovalent anions of different shapes (spherical, linear, trigonal planar and tetrahedral) and sizes (ionic radii, r, of 1.7 to 2.4ºA) were found to bind with high affinities to TK6+ in a 1:2 (TK6+: anion) stoichiometry. The first (K1), second (K2) and global binding constants (log β2) of the anions binding were reported.
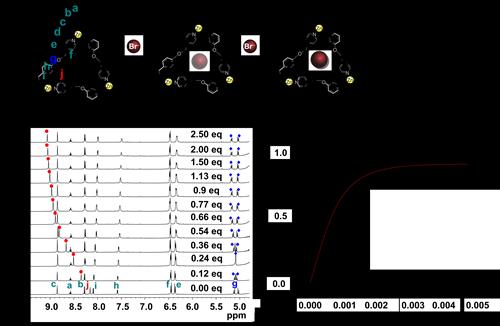
Measurement of the bromide ion binding constants of TK6+ complexes by titration. (a) Schematic representation of bromide ion binding. (b) Stacked plots of 1H NMR (600 MHz, 298 K) spectra of 1.87 mM solutions of TK6+ in D2O titrated with, bottom to top, increasing amounts of tetrabutylammonium bromide. (c) Binding isotherm obtained by plotting Hj signal shift versus bromide ion concentration. In most cases, addition of more than two equivalents of anion precipitated the knot and prevented further measurements.